

1、使用面积较小的盖玻片制作标本时,应使用ProLong Diamond Antifade Mountant 等凝固型封片剂封片。使用凝固型封片剂可以避免标本移动。
2、应尽量使用平场复消色差(Plan-APO)物镜来采集图像。使用平场复消色差(Plan-APO)物镜可以获得较平整的和色差较小的图像。
3、在采集荧光图像时尽量不要同时采集 DIC 图像以免降低图像分辨率和影响图像亮度。
4、在进行荧光亮度定量分析实验时,所有的采图参数(光路设置、激光强度、PMT 增益、PMT Offset、平均次数、Zoom 、Pinhole 等)都必须保持一致,建议使用阳性标本(荧光亮度最亮的标本)来设置参数,避免荧光亮度过饱和。应尽量预订足够长时间的实验时间,在一次实验中完成所有荧光定量分析标本的成像。
5、在共聚焦采图时,如无特殊原因 Pinhole 应该设置在 1 Airy Unit 位置,PMT Offset 应该设置在 0 位置,PMT Gain 分别设置在 50-150 (for NIKON Ti-A1R、NIKON FN1- A1R)、600-800 (for Olympus FV-1000)、40-60%(for Olympus FV-10i)、600-700 (for Zeiss LSM 510)。
6、在共聚焦活标本采图时,可以根据实验需要适当增大 Pinhole 、降低激光强度、增加 PMT Gain、降低采图分辨率、增加采图速度以减小激光对细胞活性的影响。
7、在使用 Prairie Two-photon 显微镜双光子采图时,PMT Gain 应该设置在 600-800,PMT Offset 设置在 1.17 左右,并使用 HiLo Lookup Table 检查图像亮度,避免背景过低或图像过饱和。
8、在活细胞成像时应尽量使用水镜,避免球差引起的分辨率变差、结构变形(拉长或缩短)。
9、在多标记标本荧光成像时,要使用序列扫描来减少串色。串色只能尽量减少不可能完全避免,所以还必须使用单荧光标记标本作为对照来检查是否串色, 并用软件来进行串色校正。
10、不要用 Live-scan 长时间扫描标本、也不要长时间在荧光灯下观察标本,以防标本漂白。
11、设置参数要 1 个通道 1 个通道设置,NIKON 共聚焦显微镜应使用 Preview 和 Capture 分别设置预览参数和正式采图参数。采集预览图像时应使用低分辨率扫描,不要使用高分辨率扫描或平均。
12、在多荧光标记标本成像时(尤其是使用 Plan-APO 60x 高数值孔径物镜高分辨成像时),必须使用多色荧光小球标本为对照来检查色差引起的图像在 XYZ 方向上的偏移。色差只能尽量避免(如:使用 Plan-APO 物镜、使用波长比较接近的488 nm 和561 nm 激光来采图等)不可能完全避免。色差一般为 几十-几百 纳米(视不同的显微镜构架、物镜类型、激发光波长的不同而不同),高分辨率成像实验的图像必须使用软件来进行色差校正。
13、高分辨率图像采集和活细胞图像采集的图像(包括Z-stack、Time-series、Two-photon image), 可以使用反卷积软件来进一步提高分辨率或降低噪声(提高信噪比)。反卷积的效果与原始图像的采图分辨率密切相关,一般采图分辨率需要达到物镜极限分辨率的 1/3-1/4。
图像的分辨率和信噪比对荧光共定位分析至关重要。荧光共定位分析实验必须使用高数值孔径物镜成像。图像必须经过反卷积、色差校正、串色校正等处理。需要做反卷积处理的同学请找胡谦老师讨论实验和确定采图参数。
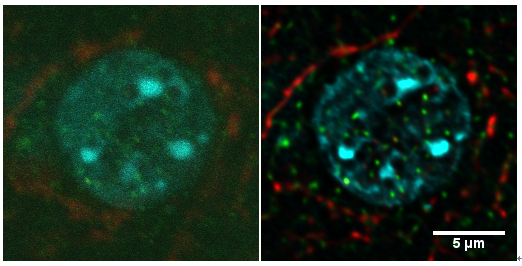
左图为原始图像(此标本荧光标记比较弱,无法获得高信噪比的图像), 右图为经过反卷积和色差校正后的图像,使用 OLYMPUS FV-1000 PLAPON 60xOil NA. 1.42 物镜, 采图分辨率 xy=0.069 um/pixel, z=0.25 um/pixel
14、 所有光学成像实验都必须设计和完成正对照实验和负对照实验来保证光学成像实验结果的正确性和准确性。http://en.wikipedia.org/wiki/Scientific_control
Positive controls are groups where a phenomenon is expected. That is, they ensure that there is an effect when there should be an effect, by using an experimental treatment that is already known to produce that effect (and then comparing this to the treatment that is being investigated in the experiment).
Negative controls are groups where no phenomenon is expected. They ensure that there is no effect when there should be no effect. To continue with the example of drug testing, a negative control is a group that has not been administered the drug of interest. This group receives either no preparation at all or a sham preparation (that is, a placebo), either an excipient-only (also called vehicle-only) preparation or the proverbial "sugar pill." We would say that the control group should show a negative or null effect.
-----------------------------------------------------------------
附:Fura-2 AM Loading Protocol
分 子 式: C44H47N3O24
分 子 量: 1001.85
外 观: 黄色或橙黄色粉末
Loading cells with Fura-2
------------------------------------------------------------------
General Information on Fura-2 AM ester:
Fura-2 is a high-affinity (Kd = 220 nM) Ca2+ dye.
AM ester = acetoxymethyl ester; The AM ester makes the dye membrane permeable because it caps hydrophilic carboxyl groups to form esters. Once the dye is taken up into cells, the esters are cleaved by intracellular esterases and the dye is trapped inside.
Excitation ratiometric imaging of Fura-2 is carried out by using 340/26 nm and 380/10 nm excitation filters, a 455 nm dichroic mirror (must have good reflectivity down to approximately 330 nm), and a 535/40 nm emission filter.
Fura-2 is light sensitive so store in lightproof manner (e.g., wrapped in tin-foil) at – 20 oC.
Both Fura-2 and Pluronic F-127 can