发布时间:2023-04-11
An embryo-like ball of cells offers a way to study pregnancy and its complications without the typical ethical dilemmas.
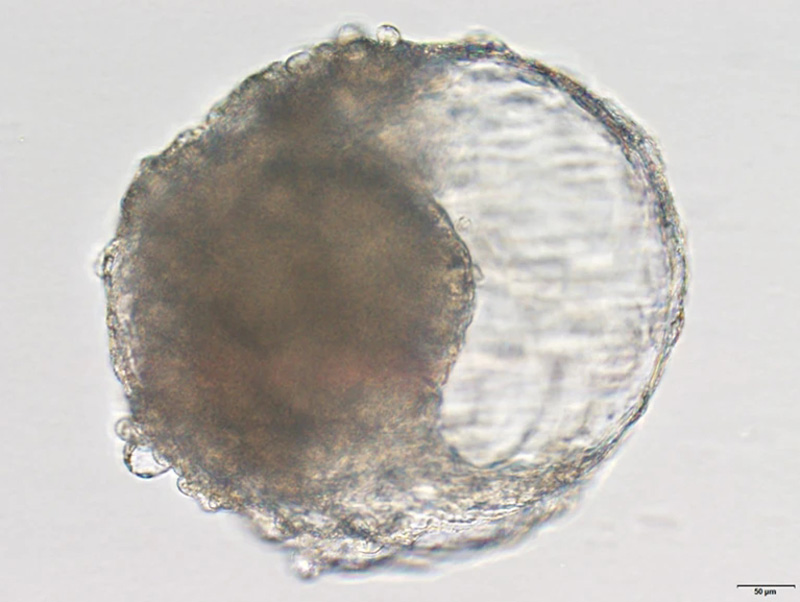
A blastoid created from cynomolgus monkey stem cells. The blastoid resembles the early stages of an embryo and triggered pregnancy-like changes when implanted in a monkey's uterus.Credit: Jie Li
Scientists have created balls of cells that resemble embryos and trigger signs of early pregnancy in macaques. The stem-cell-derived blastoids could help researchers understand human embryo development without the ethical dilemmas of using real embryonic cells, according to a study published today in Cell Stem Cell1.
“The work highlights the amazing potential of stem-cell- based embryo models as a means to explore embryonic stages that are typically difficult to access in vivo,” says Naomi Moris, a developmental biologist at the Francis Crick Institute in London.
The mechanisms of human embryo development are still largely unstudied, owing to the ethical difficulties of sourcing and experimenting with human embryos. In the laboratory, researchers have previously generated blastoids — balls of cells that resemble blastocysts, the clusters of dividing cells that form about five to six days after fertilization. In March 2021, for instance, a team led by Leqian Yu, a developmental biologist at the University of Texas Southwestern Medical Center in Dallas, successfully produced blastoids from human stem cells and developed them for ten days in a culture dish2.
Monkey models
Zhen Liu, a developmental biologist at the Institute of Neuroscience at the Chinese Academy of Sciences in Shanghai, and his colleagues turned to cynomolgus monkeys (Macaca fascicularis), otherwise known as crab-eating macaques, which are commonly used as lab animals because they have some biological similarities with humans. In cell culture, the researchers exposed monkey embryonic stem cells to various growth factors so they would differentiate into cell types found in natural blastocysts.
After about a week, the stem cells had formed the signature spherical structure of a blastocyst and had differentiated into the three cell lineages that lay the foundation for tissues and organs to form. "This has not even been achieved in the study of in vitro prolonged culture of natural monkey blastocysts," the authors say.
When the researchers profiled roughly 6,000 individual cells using single-cell RNA sequencing, they found genetic characteristics similar to those seen in natural blastocysts. Some cells expressed genes associated with the endoderm — the innermost layer that eventually forms the linings of the respiratory and gastrointestinal tracts — whereas others were enriched with genes involved in placental development. But the blastoids weren’t a perfect reflection of natural blastocysts — some cells could not be classified, and some didn’t express as much protein as expected.
By day 15, the researchers could see what looked like an outline of the yolk sac, which provides nutrition before the placenta forms, and the amnion, the outer membrane that surrounds the developing embryo. Of the 41 blastoids, 5 also developed features that resembled the primitive streak, a structure that marks the beginning of the cells rearranging themselves to form the layout of the body, including its left–right, top–bottom configuration.
Liu and his team then transferred the blastoids into the uteri of eight cynomolgus monkeys using laparoscopic surgery. For seven to ten days after the transplant, three of the monkeys had gestational sacs — fluid-filled cavities that are the first features seen by ultrasound during pregnancy. The implantation also resulted in the release of the pregnancy hormones progesterone and chorionic gonadotropin. But these were short-lived, with the blastoids and signs of pregnancies disappearing after 20 days.
In utero insights
Although the embryo-like structures were not designed to develop into full-blown fetuses, their lack of staying power in vivo indicates there is more to embryo development than having the right cells, structure and gene expression, says Alfonso Martinez Arias, a developmental biologist at Pompeu Fabra University in Barcelona, Spain.
Furthermore, embryo models that fall apart in the womb could offer insights about the factors that lead to miscarriages and implantation failure after in vitro fertilization treatments, says Alexandra Harvey, a developmental biologist at the University of Melbourne in Australia. “If these models are actually failing at that stage, we’ve got to work out what other markers we need to be looking for at earlier stages,” she says.
Stem-cell based embryo models could also be useful for teasing apart the fundamentals of embryogenesis, says Moris. By manipulating gene expression or tweaking signalling pathways in blastoids, researchers could investigate which features are necessary for embryo development, she says. Moris adds that lab-made embryo models could also be used in experiments that require large samples — such as those in proteomics — because they can be generated at scale. As embryo models become more sophisticated, governance and regulatory frameworks will need to keep pace, along with discussions about how they should be used.
The next step for Liu and his colleagues is to improve their methods for producing monkey blastoids. "This will help to achieve further embryogenesis," the authors say.
Source:Gemma Conroy 06 April 2023
 附件下载:
附件下载: